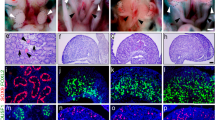
Abstract
Ferrous iron (Fe2+) is essential in all eukaryotic cells for various oxidoreductase reactions, including the demethylation of DNA and proteins. Histone demethylation is required for normal epigenetic regulation of the Y-chromosomal sex-determining gene Sry in developing gonads during male sex determination1,2. Here we investigate the potential connection between iron metabolism, histone demethylation and sex determination in mammals. We found that Fe2+-producing pathways are substantially activated in mouse embryonic gonads during the sex-determining period. Chelation of iron in cultured XY gonads reduced the level of KDM3A-mediated H3K9 demethylation of Sry, mostly abolished Sry expression and caused the gonads to express ovarian markers. In vivo, conditional deletion of the gene Tfrc—which is required for iron incorporation—in fetal XY gonadal somatic cells, or acute pharmaceutical suppression of available iron in pregnant mice, resulted in male-to-female gonadal sex reversal in a proportion of offspring, highlighting the pivotal role of iron metabolism in male sex determination. Finally, long-term feeding of pregnant mice with a low-iron diet, when combined with a heterozygous variant of Kdm3a that by itself has no observable effect, suppressed Sry expression and caused male-to-female sex reversal in some of the progeny, revealing a connection between maternal dietary iron and fetal developmental outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The scRNA-seq data are available at the GEO under GSE184708 (ref. 8) and GSE143356 (ref. 9). The RNA-seq data have been uploaded to the GEO under GSE287387. All other data are available in the manuscript or its Supplementary Information. Source data are provided with this paper.
References
Kuroki, S. et al. Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science 341, 1106–1109 (2013).
Kuroki, S. et al. Rescuing the aberrant sex development of H3K9 demethylase Jmjd1a-deficient mice by modulating H3K9 methylation balance. PLoS Genet. 13, e1007034 (2017).
Sinclair, A. H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990).
Koopman, P. et al. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121 (1991).
Kashimada, K. & Koopman, P. Sry: the master switch in mammalian sex determination. Development 137, 3921–3930 (2010).
Stévant, I. et al. Deciphering cell lineage specification during male sex determination with single-cell RNA sequencing. Cell Rep. 22, 1589–1599 (2018).
Galy, B., Conrad, M. & Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25, 133–155 (2024).
Mayère, C. et al. Origin, specification and differentiation of a rare supporting-like lineage in the developing mouse gonad. Sci. Adv. 8, eabm0972 (2022).
Guo, J. et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 28, 764–778 (2021).
Hirayama, T., Okuda, K. & Nagasawa, H. A highly selective turn-on fluorescent probe for iron(ii) to visualize labile iron in living cells. Chem. Sci. 4, 1250–1256 (2013).
Qian, Z. M., Li, H., Sun, H. & Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 54, 561–587 (2002).
Levy, J. E., Jin, O., Fujiwara, Y., Kuo, F. & Andrews, N. C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 21, 396–399 (1999).
Han, Z. et al. Iron homeostasis determines fate of human pluripotent stem cells via glycerophospholipids–epigenetic circuit. Stem Cells 37, 489–503 (2019).
Kidokoro, T. et al. Influence on spatiotemporal patterns of a male-specific Sox9 activation by ectopic Sry expression during early phases of testis differentiation in mice. Dev. Biol. 278, 511–525 (2005).
Takase, S. et al. A specific G9a inhibitor unveils BGLT3 lncRNA as a universal mediator of chemically induced fetal globin gene expression. Nat. Commun. 14, 23 (2023).
Harima, H. et al. Deferasirox, a novel oral iron chelator, shows antiproliferative activity against pancreatic cancer in vitro and in vivo. BMC Cancer 16, 702 (2016).
Eggers, S. & Sinclair, A. Mammalian sex determination—insights from humans and mice. Chromosome Res. 20, 215–238 (2012).
Munger, S. C. & Capel, B. Sex and the circuitry: progress toward a systems-level understanding of vertebrate sex determination. Wiley Interdiscip. Rev. Syst. Biol. Med. 4, 401–412 (2012).
Bashamboo, A. & McElreavey, K. Human sex-determination and disorders of sex-development (DSD). Semin. Cell Dev. Biol. 45, 77–83 (2015).
Capel, B. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 18, 675–689 (2017).
Okashita, N., Maeda, R. & Tachibana, M. CDYL reinforces male gonadal sex determination through epigenetically repressing Wnt4 transcription in mice. Proc. Natl Acad. Sci. USA 120, e2221499120 (2023).
Wilkinson, N. & Pantopoulos, K. The IRP/IRE system in vivo: insights from mouse models. Front. Pharmacol. 5, 176 (2014).
Zhou, Z. D. & Tan, E. K. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol. Neurodegener. 12, 75 (2017).
Beyer, S., Kristensen, M. M., Jensen, K. S., Johansen, J. V. & Staller, P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J. Biol. Chem. 283, 36542–36552 (2008).
Krieg, A. J. et al. Regulation of the histone demethylase JMJD1A by hypoxia-inducible factor 1α enhances hypoxic gene expression and tumor growth. Mol. Cell. Biol. 30, 344–353 (2010).
Mimura, I. et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol. Cell. Biol. 32, 3018–3032 (2012).
Hoefele, J. et al. Disorders of sex development and Diamond–Blackfan anemia: is there an association? Pediatr. Nephrol. 25, 1255–1261 (2010).
Granada, M. L. & Audi, L. The laboratory in the multidisciplinary diagnosis of differences or disorders of sex development (DSD): (III) Biochemical and genetic markers in the 46,XY; (IV) Proposals for the differential diagnosis of DSD. Adv. Lab. Med. 2, 494–504 (2021).
Hashimoto, M. & Takemoto, T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci. Rep. 5, 11315 (2015).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Okashita, N. et al. PRDM14 promotes active DNA demethylation through the ten-eleven translocation (TET)-mediated base excision repair pathway in embryonic stem cells. Development 141, 269–280 (2014).
Maeda, R. & Tachibana, M. HP1 maintains protein stability of H3K9 methyltransferases and demethylases. EMBO Rep. 23, e53581 (2022).
Okashita, N., Kuroki, S., Maeda, R. & Tachibana, M. TET2 catalyzes active DNA demethylation of the Sry promoter and enhances its expression. Sci. Rep. 9, 13462 (2019).
Miyawaki, S. et al. The mouse Sry locus harbors a cryptic exon that is essential for male sex determination. Science 370, 121–124 (2020).
Mukaide, T. et al. Histological detection of catalytic ferrous iron with the selective turn-on fluorescent probe RhoNox-1 in a Fenton reaction-based rat renal carcinogenesis model. Free Radic. Res. 48, 990–995 (2014).
Matoba, S. et al. Establishment of testis-specific SOX9 activation requires high-glucose metabolism in mouse sex differentiation. Dev. Biol. 324, 76–87 (2008).
Sasaki, K. et al. The embryonic ontogeny of the gonadal somatic cells in mice and monkeys. Cell Rep. 35, 109075 (2021).
Acknowledgements
The authors thank the members of the M.T. laboratory for technical support and discussions; M. Saitou for Wt1creERT2 mice; Y. Kanai for Hsp-Sry-tg mice; J. Sakai for the KDM3A antibody; H. Kimura for histone-modified antibodies; and A. Ito for RK-701. The authors acknowledge the NGS core facility of the Genome Information Research Center at the Research Institute for Microbial Diseases of Osaka University for support with RNA-seq and data analysis. This study was supported by the Chubei Itoh Foundation (N.O.), the Tojuro Iijima Foundation for Food Science and Technology (N.O.), the Food Science Institute Foundation (N.O.), the Terumo Life Science Foundation (M.T.), the Uehara Memorial Foundation (M.T) and JSPS KAKENHI (grant numbers 23K05732 (N.O.), 23K18139 (S.K.), 23K27182 (S.K.), 24H01368 (S.K.), 17H06424 (M.T), 21K19221 (M.T) and 24H02320 (M.T)).
Author information
Authors and Affiliations
Contributions
N.O. and M.T. conceived the project. N.O., P.K. and M.T. interpreted data and wrote the manuscript. N.O. performed most of the experiments. R.M. performed all bioinformatic data analysis. S.K. performed RNA-expression analysis in Sry-expressing pre-Sertoli cells. K.S. supported the analysis and visualization of RNA-seq data. Y.U. contributed to the establishment of Tfrc-mutant mice and the observation of postnatal mouse gonads. All authors edited and provided comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Iron-metabolism-related genes are activated in pre-Sertoli cells.
a, Schematic representation for the isolation of NR5A1+ gonadal somatic cells. Gonadal somatic cells expressing the cell surface marker protein CD271, under the control of a CD271 transgene driven by the Nr5a1 promoter, were immunomagnetically isolated from gonad/mesonephros as described previously1. b, Gene-expression profiles of NR5A1+ and NR5A1− cells examined by quantitative mRNA expression analysis. Schematic illustration of mitochondrial iron metabolism (left). Slc25a37/MFRN1 and Slc25a28/MFRN2 import Fe2+ from the intermembrane space of the mitochondria. Flvcr1 gene encodes FLVCR1A and FLVCR1B. FLVCR1B, locales in the mitochondria, regulates haem export into the cytosol. Ftmt/FTMT catalyses the oxidation of Fe2+ to Fe3+ and stores iron. NR5A1+ and NR5A1− cells were separated from XY gonad/mesonephros at E11.5, and then applied into quantitative mRNA expression analysis. Expression profiles of Slc25a37, Slc25a28, Flvcr1, and Ftmt are shown on the right. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d. All differences between means with P < 0.01 are indicated; two-tailed paired t-tests. c, Box-and-whisker plot showing expression (log-normalized counts) of Nr5a1, Kdm3a, Sry, Tfrc, Scara5, Slc11a2, Steap3, Ncoa4, Hmox1, Slc40a1, and Heph of XX or XY gonads in annotated cell populations. The populations were Coelomic epithelial cells, Endothelial cells, Germ cells, Mesonephric mesenchymal cells, Mesonephric tubules, pre-Supporting cells, and Supporting-like cells. Data were extracted from scRNA-seq analysis of XX or XY embryos at E11.5 (GEO: GSE184708). Numbers of cells examined are shown in the legend. Boxes encompass the interquartiles (25th–75th percentiles), The horizontal lines are medians, dots are outliers, and range bars show the maximum and minimum values excluding outliers. d, Comparison of expression levels of KDM3A, SRY, and iron-metabolism genes in human XY NR5A1+ and NR5A1− cells. Data were extracted from scRNA-seq analysis of human embryonic stages at 6, 7, 8, and 12 weeks post-fertilization (GEO: GSE143356). *P < 0.05, **P < 0.01; Wilcoxon rank sum test. The embryonic stages from 6 to 8 weeks post-fertilization are highlighted as the sex-determining period.
Extended Data Fig. 2 Effect of Tfrc deficiency on gonadal sex development.
a, Experimental scheme to generate Tfrc deletion allele. Protein and genomic structures of TFR1/Tfrc are represented at the top. The position of gRNA target sites is indicated in yellow. Sequence with knock-in two loxP sites (red) and sequence cleaved by Cre recombinase are shown in the middle and bottom, respectively. b, Immunoblot analysis to compare TFR1 expression in E9.5 whole embryos of the indicated genotypes. GAPDH is used as an internal control. c, Immunoblot analysis to compare TFR1 and H-Ferritin expression in E11.5 NR5A1+ cells of the indicated genotypes. The relative values were calculated based on the signal of α-tubulin. d, Effect of Tfrc deficiency on the number of gonadal somatic cells in developing embryos. NR5A1+ cells were isolated from E11.5 embryos of the indicated genotypes and counted. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d. e, Expression profiles of Sry, Scara5, Slc11a2, Steap3, Ncoa4 and Hmox1 in E11.5 NR5A1+ cells of the indicated genotypes. NR5A1+ cells were purified from E11.5 embryos and subjected to quantitative RT–qPCR analysis. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d. All differences between means with P < 0.01 are indicated; two-tailed paired t-tests. f, External genitalia (Upper) and internal genitalia (Lower) of mice of the indicated genotypes. Arrowheads indicate mammary glands. The age of the mouse is presented in the lower left corner. Frequencies of female-to-male sex reversal are presented in the lower right corner. Ov, Ovary. Sex development frequencies are shown in the lower right corner. Red bar indicates mice carrying two ovaries. The number of mice examined is shown above the bars.
Extended Data Fig. 3 Effect of DFO treatment on gonadal sex development in in-vitro-cultured gonads.
a, Development an in vitro culture system to monitor gonadal sex development. Pairs of aorta/gonad/mesonephros/mesenchyme were isolated from E10.8 XY or XX embryos, and then cultured for 14.4 h, 19.2 h and 24 h. NR5A1+ cells were isolated from XY gonads at each time point, and then applied into quantitative mRNA expression analysis. Sry expression reached a peak after 19.2 h of culture. Further 24 h couture (total 43.2 h culture) gave rise to sexually differentiated gonads with either male-type SOX9+ cells or female-type FOXL2+ cells. b, Effect of DFO treatment on the number of gonadal somatic cells in in vitro cultured gonads. NR5A1+ cells were isolated from XY gonads after 19.2-h incubation. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d. c, Compared expression levels of H/L-Ferritin between DFO-treated and untreated gonadal somatic cells. NR5A1+ cells were isolated from XY gonads after 19.2-h incubation and introduced into immunoblot. The relative values were calculated based on the signal of α-Tubulin. d, PCA of mRNA expression in NR5A1+ cells from in vitro cultured gonads (19.2 h culture, labelled as IVC) and in utero developed gonads (labelled as E11.5). e, mRNA heat map of genes involved in gonadogenesis and/or Sry regulation in NR5A1+ cells from in vitro cultured gonads (19.2 h, labelled as IVC) and in utero developed gonads (labelled as E11.5). f, Venn diagram illustrates the overlap between genes downregulated by DFO treatment in in vitro cultured NR5A1+ cells and KDM3A target genes in in utero developed NR5A1+ cells. Blue circle indicates 2677 genes downregulated in DFO-treated NR5A1+ cells (TPM > 0; fold change > 2). Among the 131 KDM3A target genes in in utero developed NR5A1+ cells1, 100 genes were detected in in vitro cultured gonads (TPM > 0, indicated in red circle). Merged 40 genes represent KDM3A target genes that were downregulated in DFO-treated NR5A1+ cells. g, RNA-seq based gene-expression values (TPM) of JmjC family in in vitro cultured gonads. NR5A1+ cells were isolated from XY gonads after 19.2-h incubation. Data are presented as mean ± SD. All differences between means with FDR < 0.01 are indicated. h, Compared protein expression levels of KDM3A between DFO-treated and untreated gonadal somatic cells. NR5A1+ cells were isolated from XY gonads after 19.2-h incubation and introduced into immunoblot. The relative values were calculated based on the signal of GAPDH. i, Compared H3K9me2 levels between DFO-treated and untreated gonadal somatic cells. NR5A1+ cells were isolated from XY and XX gonads after 19.2-h incubation and introduced into immunoblot. The relative values were calculated based on the signal of H3.1/2. j, Quantification of H3K9me2 levels at the KDM3A target gene loci Sry, Myl7, and Apoc1 in DFO-treated gonadal somatic cells. NR5A1+ cells were isolated from XY gonads after 19.2-h incubation and introduced into ChIP–qPCR. ChIP experiment was performed independently three times. Data are presented as mean ± s.d. All differences between means with P < 0.01 are indicated; two-tailed paired t-tests.
Extended Data Fig. 4 Induction of maternal–fetal anaemia by the iron chelator DFX.
a, Immunoblot analysis of H/L-ferritin in E11.5 XY NR5A1+ cells in embryos carried by DFX-treated mothers and those carried by control mothers. GAPDH is used as an internal control. b, Effect of treating pregnant mothers with DFX an the number of gonadal somatic cells in developing embryos in utero. NR5A1+ cells were isolated from E11.5 XY embryos carried by DFX-treated mothers and those carried by control mothers. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d. c, Expression profiles of Sry, Nr5a1, Gata4, Wt1, Kdm3a, Tfrc, Hmox1, Myl7, and Apoc1 in E11.5 XY NR5A1+ cells in the embryos carried by DFX-treated mothers and those carried by control mothers. NR5A1+ cells were purified from E11.5 embryos and subjected to RT–qPCR analysis. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d. All differences between means with P < 0.01 are indicated; two-tailed paired t-tests.
Extended Data Fig. 5 Induction of maternal–fetal anaemia by an IDD.
a, Changes in body weight (left), haemoglobin levels (middle), and red blood cells (right) in IDD-fed mothers and CD-fed mothers were monitored during the pre-feeding (0–4 week) and gestation (4–6 week) periods. Numbers of examined female mice are shown above the markers. Data are presented as mean ± s.d. *P < 0.05, ***P < 0.001; two-tailed paired t-tests. b, Giemsa staining of a peripheral blood smear from pregnant IDD-fed mothers and CD-fed mothers when embryos were at E11.5. c, Immunoblot analysis of TFR1 and H/L-Ferritin in E11.5 XY gonadal NR5A1+ cells in the embryos carried by IDD-fed mothers and CD-fed mothers. GAPDH is used as an internal control. d, Effect of mother’s iron deficiency anaemia induced by IDD feeding on the number of gonadal somatic cells in the corresponding embryos. NR5A1+ cells were isolated from E11.5 XY gonads in the embryos carried by IDD-fed mothers and those carried by CD-fed mothers and counted. Numbers of embryos examined are shown above the bars. Data are presented as mean ± s.d.
Supplementary information
Supplementary Figure 1
Uncropped images of immunoblots. Ponceau S staining and uncropped blot images corresponding to the main figures
Supplementary Table 1
Gene-expression values (TPM) from RNA-seq analysis. Summary of TPM values for genes examined in the RNA-seq dataset
Supplementary Table 2
Primers used in this study. Nucleotide sequence information of primer sets used in this study
Supplementary Table 3
Antibodies used in this study. Information on antibodies used in this study
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okashita, N., Maeda, R., Kuroki, S. et al. Maternal iron deficiency causes male-to-female sex reversal in mouse embryos. Nature (2025). https://doi.org/10.1038/s41586-025-09063-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-025-09063-2