Abstract
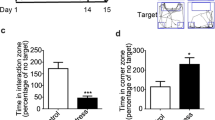
Hyponatremia is the most common clinical electrolyte disorder. Once thought to be asymptomatic in response to adaptation by the brain, recent evidence suggests that chronic hyponatremia (CHN) may induce neurological manifestations, including psychological symptoms. However, the specific psychological symptoms induced by CHN, the mechanisms underlying these symptoms, and their potential reversibility remain unclear. Therefore, this study aimed to determine whether monoaminergic neurotransmission is associated with innate anxiety-like behaviors potentiated by CHN in a mouse model of CHN secondary to the syndrome of inappropriate antidiuresis. In the present study, using a mouse model of the syndrome of inappropriate antidiuresis presenting with CHN, we showed that the sustained reduction of serum sodium ion concentrations potentiated innate anxiety-like behaviors in the light/dark transition and open field tests. We also found that serotonin and dopamine levels in the amygdala were significantly lower in mice with CHN than in controls. Additionally, phosphorylation of extracellular signal-regulated kinase (ERK) in the amygdala was significantly reduced in mice with CHN. Notably, after correcting for CHN, the increased innate anxiety-like behaviors, decreased serotonin and dopamine levels, and reduced phosphorylation of ERK in the amygdala were normalized. These findings further underscore the importance of treating CHN and highlight potential therapeutic strategies for alleviating anxiety in patients with CHN, which will improve their quality of life.








Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ (2013) Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med 126(3):256–263. https://doi.org/10.1016/j.amjmed.2012.06.037
Fenske W, Maier SK, Blechschmidt A, Allolio B, Stork S (2010) Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med 123(7):652–657. https://doi.org/10.1016/j.amjmed.2010.01.013
Hannon MJ, Thompson CJ (2010) The syndrome of inappropriate antidiuretic hormone: prevalence, causes and consequences. Eur J Endocrinol 162(Suppl 1):S5-12. https://doi.org/10.1530/EJE-09-1063
Adrogue HJ, Madias NE (2000) Hyponatremia. N Engl J Med 342(21):1581–1589. https://doi.org/10.1056/NEJM200005253422107
Verbalis JG (2010) Brain volume regulation in response to changes in osmolality. Neuroscience 168(4):862–870. https://doi.org/10.1016/j.neuroscience.2010.03.042
Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G (2006) Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 119(1):71–78
Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G (2008) Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 101(7):583–588. https://doi.org/10.1093/qjmed/hcn061
Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, Resnick HE (2010) Hyponatremia-induced osteoporosis. J Bone Miner Res 25(3):554–563. https://doi.org/10.1359/jbmr.090827
Gunathilake R, Oldmeadow C, McEvoy M, Kelly B, Inder K, Schofield P, Attia J (2013) Mild hyponatremia is associated with impaired cognition and falls in community-dwelling older persons. J Am Geriatr Soc 61(10):1838–1839. https://doi.org/10.1111/jgs.12468
Lee S, Min JY, Kim B, Ha SW, Han JH, Min KB (2021) Serum sodium in relation to various domains of cognitive function in the elderly US population. BMC Geriatr 21(1):328. https://doi.org/10.1186/s12877-021-02260-4
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C, Investigators S (2006) Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355(20):2099–2112. https://doi.org/10.1056/NEJMoa065181
Fujisawa C, Umegaki H, Sugimoto T, Samizo S, Huang CH, Fujisawa H, Sugimura Y, Kuzuya M et al (2021) Mild hyponatremia is associated with low skeletal muscle mass, physical function impairment, and depressive mood in the elderly. BMC Geriatr 21(1):15. https://doi.org/10.1186/s12877-020-01955-4
Fujisawa H, Sugimura Y, Takagi H, Mizoguchi H, Takeuchi H, Izumida H, Nakashima K, Ochiai H et al (2016) Chronic Hyponatremia Causes Neurologic and Psychologic Impairments. J Am Soc Nephrol 27(3):766–780. https://doi.org/10.1681/ASN.2014121196
Verbalis JG, Gullans SR (1991) Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res 567(2):274–282. https://doi.org/10.1016/0006-8993(91)90806-7
Gankam Kengne F, Decaux G (2018) Hyponatremia and the Brain. Kidney Int Rep 3(1):24–35. https://doi.org/10.1016/j.ekir.2017.08.015
Babaev O, Piletti Chatain C, Krueger-Burg D (2018) Inhibition in the amygdala anxiety circuitry. Exp Mol Med 50(4):1–16. https://doi.org/10.1038/s12276-018-0063-8
Tovote P, Fadok JP, Luthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16(6):317–331. https://doi.org/10.1038/nrn3945
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517(7534):284–292. https://doi.org/10.1038/nature14188
Steinbusch HW (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6(4):557–618. https://doi.org/10.1016/0306-4522(81)90146-9
Baratta MV, Kodandaramaiah SB, Monahan PE, Yao J, Weber MD, Lin PA, Gisabella B, Petrossian N et al (2016) Stress Enables Reinforcement-Elicited Serotonergic Consolidation of Fear Memory. Biol Psychiatry 79(10):814–822. https://doi.org/10.1016/j.biopsych.2015.06.025
Yu XD, Zhu Y, Sun QX, Deng F, Wan J, Zheng D, Gong W, Xie SZ et al (2022) Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety. Nat Neurosci 25(12):1651–1663. https://doi.org/10.1038/s41593-022-01200-8
Matthiesen M, Mendes LD, Spiacci A Jr, Fortaleza EA, Correa FM, Zangrossi H Jr (2020) Serotonin 2C receptors in the basolateral amygdala mediate the anxiogenic effect caused by serotonergic activation of the dorsal raphe dorsomedial subnucleus. J Psychopharmacol 34(4):391–399. https://doi.org/10.1177/0269881119882797
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR (2008) Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 33(13):3221–3225. https://doi.org/10.1038/npp.2008.52
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ (2009) Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194(6):535–540. https://doi.org/10.1192/bjp.bp.108.056093
de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K (2010) Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol 90(2):198–216. https://doi.org/10.1016/j.pneurobio.2009.10.010
Asan E (1998) The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol 142:1–118. https://doi.org/10.1007/978-3-642-72085-7
Morel C, Montgomery SE, Li L, Durand-de Cuttoli R, Teichman EM, Juarez B, Tzavaras N, Ku SM et al (2022) Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Nat Commun 13(1):1532. https://doi.org/10.1038/s41467-022-29155-1
McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR (2017) Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife 6:e18247. https://doi.org/10.7554/eLife.18247
Siuda ER, Al-Hasani R, McCall JG, Bhatti DL, Bruchas MR (2016) Chemogenetic and Optogenetic Activation of Galphas Signaling in the Basolateral Amygdala Induces Acute and Social Anxiety-Like States. Neuropsychopharmacology 41(8):2011–2023. https://doi.org/10.1038/npp.2015.371
Kawakami T, Fujisawa H, Nakayama S, Yoshino Y, Hattori S, Seino Y, Takayanagi T, Miyakawa T et al (2021) Vasopressin escape and memory impairment in a model of chronic syndrome of inappropriate secretion of antidiuretic hormone in mice. Endocr J 68(1):31–43. https://doi.org/10.1507/endocrj.EJ20-0289
Crawley J, Goodwin FK (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13(2):167–170. https://doi.org/10.1016/0091-3057(80)90067-2
Takao K, Miyakawa T (2006) Light/dark transition test for mice. J Vis Exp 1:104. https://doi.org/10.3791/104
Shoji H, Ikeda K, Miyakawa T (2023) Behavioral phenotype, intestinal microbiome, and brain neuronal activity of male serotonin transporter knockout mice. Mol Brain 16(1):32. https://doi.org/10.1186/s13041-023-01020-2
Cabib S, Algeri S, Perego C, Puglisi-Allegra S (1990) Behavioral and biochemical changes monitored in two inbred strains of mice during exploration of an unfamiliar environment. Physiol Behav 47(4):749–753. https://doi.org/10.1016/0031-9384(90)90089-m
Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L (2000) Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behav Genet 30(4):285–293. https://doi.org/10.1023/a:1026545316455
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463(1–3):3–33. https://doi.org/10.1016/s0014-2999(03)01272-x
Lu Q, Mouri A, Yang Y, Kunisawa K, Teshigawara T, Hirakawa M, Mori Y, Yamamoto Y et al (2019) Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav Brain Res 372:112053. https://doi.org/10.1016/j.bbr.2019.112053
Kubota H, Kunisawa K, Niijima M, Hirakawa M, Mori Y, Hasegawa M, Fujigaki S, Fujigaki H, Y (2022) Deficiency of kynurenine 3-monooxygenase exacerbates impairment of prepulse inhibition induced by phencyclidine. Biochem Biophys Res Commun 629:142–151. https://doi.org/10.1016/j.bbrc.2022.09.003
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
de Carvalho CR, Lopes MW, Constantino LC, Hoeller AA, de Melo HM, Guarnieri R, Linhares MN, Bortolotto ZA et al (2021) The ERK phosphorylation levels in the amygdala predict anxiety symptoms in humans and MEK/ERK inhibition dissociates innate and learned defensive behaviors in rats. Mol Psychiatry 26(12):7257–7269. https://doi.org/10.1038/s41380-021-01203-0
Canteras NS, Resstel LB, Bertoglio LJ, Carobrez Ade P, Guimaraes FS (2010) Neuroanatomy of anxiety. Curr Top Behav Neurosci 2:77–96. https://doi.org/10.1007/7854_2009_7
Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG (2008) Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci U S A 105(4):1333–1338. https://doi.org/10.1073/pnas.0711496105
Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S et al (2011) SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 147(7):1459–1472. https://doi.org/10.1016/j.cell.2011.10.054
Faria R, Magalhaes A, Monteiro PR, Gomes-Da-Silva J, Amelia Tavares M, Summavielle T (2006) MDMA in adolescent male rats: decreased serotonin in the amygdala and behavioral effects in the elevated plus-maze test. Ann N Y Acad Sci 1074:643–649. https://doi.org/10.1196/annals.1369.062
Dimonte S, Sikora V, Bove M, Morgese MG, Tucci P, Schiavone S, Trabace L (2023) Social isolation from early life induces anxiety-like behaviors in adult rats: Relation to neuroendocrine and neurochemical dysfunctions. Biomed Pharmacother 158:114181. https://doi.org/10.1016/j.biopha.2022.114181
Benvenuti S, Deledda C, Luciani P, Giuliani C, Fibbi B, Muratori M, Peri A (2016) Neuronal distress induced by low extracellular sodium in vitro is partially reverted by the return to normal sodium. J Endocrinol Invest 39(2):177–184. https://doi.org/10.1007/s40618-015-0352-1
Barsony J, Sugimura Y, Verbalis JG (2011) Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem 286(12):10864–10875. https://doi.org/10.1074/jbc.M110.155002
Kuhn DM, Sykes CE, Geddes TJ, Jaunarajs KL, Bishop C (2011) Tryptophan hydroxylase 2 aggregates through disulfide cross-linking upon oxidation: possible link to serotonin deficits and non-motor symptoms in Parkinson’s disease. J Neurochem 116(3):426–437. https://doi.org/10.1111/j.1471-4159.2010.07123.x
Kuhn DM, Arthur R Jr (1997) Molecular mechanism of the inactivation of tryptophan hydroxylase by nitric oxide: attack on critical sulfhydryls that spare the enzyme iron center. J Neurosci 17(19):7245–7251. https://doi.org/10.1523/JNEUROSCI.17-19-07245.1997
Zarrindast MR, Khakpai F (2015) The Modulatory Role of Dopamine in Anxiety-like Behavior. Arch Iran Med 18(9):591–603
Ko MJ, Chiang T, Mukadam AA, Mulia GE, Gutridge AM, Lin A, Chester JA, van Rijn RM (2021) beta-Arrestin-dependent ERK signaling reduces anxiety-like and conditioned fear-related behaviors in mice. Sci Signal 14:eaba0245. https://doi.org/10.1126/scisignal.aba0245
Ailing F, Fan L, Li S, Manji S (2008) Role of extracellular signal-regulated kinase signal transduction pathway in anxiety. J Psychiatr Res 43(1):55–63. https://doi.org/10.1016/j.jpsychires.2008.01.018
Maldonado NM, Espejo PJ, Martijena ID, Molina VA (2014) Activation of ERK2 in basolateral amygdala underlies the promoting influence of stress on fear memory and anxiety: influence of midazolam pretreatment. Eur Neuropsychopharmacol 24(2):262–270. https://doi.org/10.1016/j.euroneuro.2013.10.005
Adayev T, El-Sherif Y, Barua M, Penington NJ, Banerjee P (1999) Agonist stimulation of the serotonin1A receptor causes suppression of anoxia-induced apoptosis via mitogen-activated protein kinase in neuronal HN2-5 cells. J Neurochem 72(4):1489–1496. https://doi.org/10.1046/j.1471-4159.1999.721489.x
Mogha A, Guariglia SR, Debata PR, Wen GY, Banerjee P (2012) Serotonin 1A receptor-mediated signaling through ERK and PKCalpha is essential for normal synaptogenesis in neonatal mouse hippocampus. Transl Psychiatry 2(1):e66. https://doi.org/10.1038/tp.2011.58
Chen J, Rusnak M, Luedtke RR, Sidhu A (2004) D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J Biol Chem 279(38):39317–39330. https://doi.org/10.1074/jbc.M403891200
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5(3):173–183. https://doi.org/10.1038/nrn1346
Tafet GE, Nemeroff CB (2020) Pharmacological Treatment of Anxiety Disorders: The Role of the HPA Axis. Front Psychiatry 11:443. https://doi.org/10.3389/fpsyt.2020.00443
Zou H, Pu W, Zhou J, Li J, Ma L, Wang S, Liu C, Mou J et al (2025) Noradrenergic Locus Coeruleus-CA3 Activation Alleviates Neuropathic Pain and Anxiety- and Depression-Like Behaviors by Suppressing Microglial Neuroinflammation in SNI Mice. CNS Neurosci Ther 31(3):e70360. https://doi.org/10.1111/cns.70360
Kobayashi K, Shikano K, Kuroiwa M, Horikawa M, Ito W, Nishi A, Segi-Nishida E, Suzuki H (2022) Noradrenaline activation of hippocampal dopamine D(1) receptors promotes antidepressant effects. Proc Natl Acad Sci U S A 119(33):e2117903119. https://doi.org/10.1073/pnas.2117903119
Hu H, Zarate CA Jr, Verbalis J (2024) Arginine vasopressin in mood disorders: A potential biomarker of disease pathology and a target for pharmacologic intervention. Psychiatry Clin Neurosci 78(9):495–506. https://doi.org/10.1111/pcn.13703
Appenrodt E, Schnabel R, Schwarzberg H (1998) Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav 64(4):543–547. https://doi.org/10.1016/s0031-9384(98)00119-x
Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ (2004) Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29(3):483–493. https://doi.org/10.1038/sj.npp.1300360
Juul KV, Bichet DG, Nielsen S, Norgaard JP (2014) The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol 306(9):F931-940. https://doi.org/10.1152/ajprenal.00604.2013
Arampatzis S, Frauchiger B, Fiedler GM, Leichtle AB, Buhl D, Schwarz C, Funk GC, Zimmermann H et al (2012) Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med 125(11):1125. https://doi.org/10.1016/j.amjmed.2012.04.041
Verbalis JG (1993) Hyponatremia induced by vasopressin or desmopressin in female and male rats. J Am Soc Nephrol 3(9):1600–1606. https://doi.org/10.1681/ASN.V391600
Acknowledgements
We sincerely thank Asami Yamaguchi for technical assistance.
Funding
This work was supported by JSPS KAKENHI (Grant Number 20 K08919 to Yoshihisa Sugimura, 22 K16229 to Haruki Fujisawa), The Salt Science Research Foundation No. 22 C2, YOKOYAMA Foundation for Clinical Pharmacology, The Hori Science And Arts Foundation, The Nitto Foundation, and MEXT Promotion of Distinctive Joint Research Center Program (Grant Number FY2018-2020 JPMXP0618217663, FY2021-2023 JPMXP0621467949).
Author information
Authors and Affiliations
Contributions
Haruki Fujisawa; Conceptualization, Formal analysis, Funding acquisition, Investigation, Validation, Visualization, Project administration, Writing – original draft. Nobuhiko Magara: Investigation. Shogo Nakayama: Investigation. Sachiho Fuse: Investigation. Naoko Iwata: Formal analysis, Validation. Masaya Hasegawa: Investigation, Formal analysis. Hisayoshi Kubota: Investigation, Formal analysis, Writing – review & editing. Hirotaka Shoji: Methodology, Software, Writing – review & editing. Satoko Hattori: Formal analysis, Methodology, Software, Validation, Writing – review & editing. Hideo Hagihara: Methodology, Software, Writing – review & editing. Hidetsugu Fujigaki: Investigation, Methodology, Writing – review & editing. Yusuke Seino: Writing – review & editing. Akihiro Mouri: Investigation, Supervision, Writing – review & editing. Tsuyoshi Miyakawa: Funding acquisition, Supervision, Writing – review & editing. Toshitaka Nabeshima: Supervision, Writing – review & editing. Suzuki Atsushi: Supervision, Writing – review & editing. Yoshihisa Sugimura: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Corresponding authors
Ethics declarations
Ethics Approval
All procedures were performed in accordance with the institutional guidelines for animal care at Fujita Health University, Japan, which conformed to the National Institutes of Health Animal Care Guidelines and were approved by the Institutional Animal Care and Use Committee of Fujita Health University (approval number APU22094).
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved the final manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujisawa, H., Magara, N., Nakayama, S. et al. Chronic Hyponatremia Potentiates Innate Anxiety-Like Behaviors Through the Dysfunction of Monoaminergic Neurons in Mice. Mol Neurobiol (2025). https://doi.org/10.1007/s12035-025-05024-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-025-05024-y